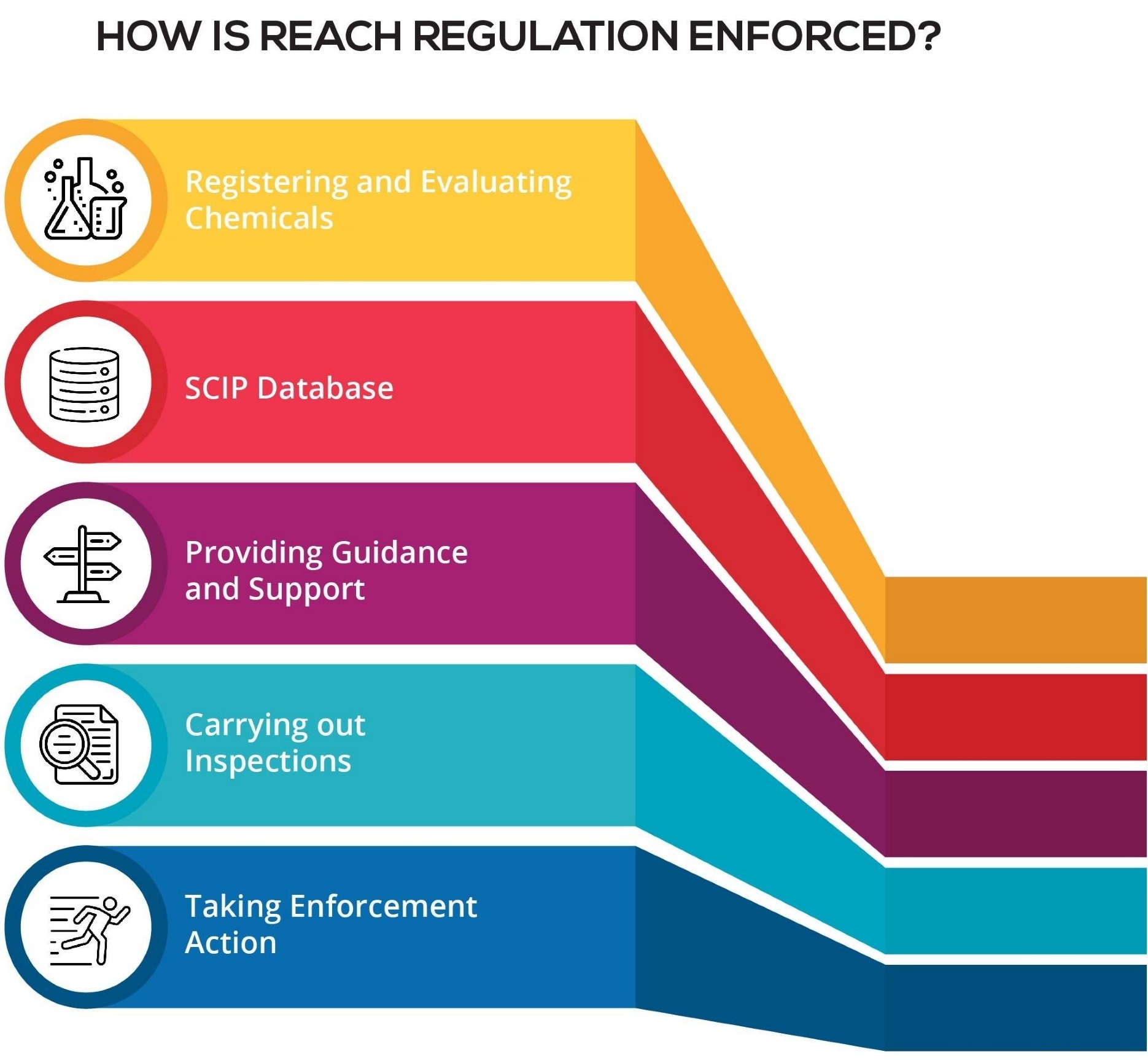
Understanding the REACH Regulation
REACH stands for Registration, Evaluation, Authorisation and Restriction of Chemicals. It entered into force on 1 June 2007.
REACH is a regulation of the European Union, adopted to improve the protection of human health and the environment from the risks that can be posed by chemicals, while enhancing the competitiveness of the EU chemicals industry. It also promotes alternative methods for the hazard assessment of substances in order to reduce the number of tests on animals. In principle, the REACH regulation applies to all chemical substances; not only those used in industrial processes but also in our day-to-day lives, for example in cleaning products, paints as well as in articles such as clothes, furniture and electrical appliances. Therefore, the regulation has an impact on most companies across the EU.
REACH places the burden of proof on companies. To comply with the regulation, companies must identify and manage the risks linked to the substances they manufacture and market in the EU. They have to demonstrate to ECHA how the substance can be safely used, and they must communicate the risk management measures to the users. If the risks cannot be managed, authorities can restrict the use of substances in different ways. In the long run, the most hazardous substances should be substituted with less dangerous ones.
How does REACH work?
REACH establishes procedures for collecting and assessing information on the properties and hazards of substances. Companies need to register their substances and to do this they need to work together with other companies who are registering the same substance. ECHA receives and evaluates individual REACH registrations for their compliance, and the EU Member States evaluate selected substances to clarify initial concerns for human health or for the environment. Authorities and ECHA’s scientific committees assess whether the risks of substances can be managed. Authorities can ban hazardous substances if their risks are unmanageable. They can also decide to restrict a use or make it subject to a prior authorisation.
The Registration Process
Companies are responsible for collecting information on the properties and uses of the substances they manufacture or import above one tonne a year. They also have to assess the hazards and potential risks presented by the substance. This information is communicated to ECHA through a registration dossier containing the hazard information and, where relevant, an assessment of the risks that the use of the substance may pose and how these risks should be controlled.
Registration applies to substances on their own, substances in mixtures and certain cases of substances in articles. Chemical substances that are already regulated by other legislations such as medicines, or radioactive substances are partially or completely exempted from REACH requirements.
Registration is based on the “one substance, one registration” principle. This means that manufacturers and importers of the same substance have to submit their registration jointly. The analytical and spectral information provided should be consistent and sufficient to confirm the substance identity.
The Evaluation Process
ECHA and the Member States evaluate the information submitted by companies to examine the quality of the registration dossiers and the testing proposals and to clarify if a given substance constitutes a risk to human health or the environment.
Evaluation under REACH focuses on three different areas:
- Examination of testing proposals submitted by registrants
- Compliance check of the dossiers submitted by registrants
- Substance evaluation
Once the evaluation is done, registrants may be required to submit further information on the substance.
In line with art. 54 of the REACH Regulation, by 28 February of each year, ECHA has to publish a report on the progress it has made over the previous calendar year on its obligations in relation to evaluation. ECHA is specifically required to include recommendations to potential registrants to foster improvement in the quality of future registrations, in these reports.
The Authorisation Process
The authorisation procedure aims to assure that the risks from Substances of Very High Concern are properly controlled and that these substances are progressively replaced by suitable alternatives while ensuring the good functioning of the EU internal market.
Substances with the following hazard properties may be identified as Substances of Very High Concern (SVHCs):
- Substances meeting the criteria for classification as carcinogenic, mutagenic or toxic for reproduction category 1A or 1B in accordance with Commission Regulation (EC) No 1272/2008 (CMR substances)
- Substances which are persistent, bioaccumulative and toxic (PBT) or very persistent and very bioaccumulative (vPvB) according to REACH (Annex XIII)
- Substances identified on a case-by-case basis, for which there is scientific evidence of probable serious effects that cause an equivalent level of concern as with CMR or PBT/vPvB substances
After a two-step regulatory process, SVHCs may be included in the Authorization List and become subject to authorization. These substances cannot be placed on the market or used after a given date, unless an authorization is granted for their specific use, or the use is exempted from authorization.
Manufacturers, importers or downstream users of a substance on the Authorization List can apply for authorization.
The Restriction Process
Restrictions are a tool to protect human health and the environment from unacceptable risks posed by chemicals. Restrictions may limit or ban the manufacture, placing on the market or use of a substance. A restriction applies to any substance on its own, in a mixture or in an article, including those that do not require registration. It can also apply to imports. A Member State, or ECHA on request of the European Commission, can propose restrictions if they find that the risks need to be addressed on an Union wide basis. ECHA can also propose a restriction on articles containing substances that are in the Authorization list (Annex XIV).
Anyone can comment on a proposal to restrict a substance. Those most likely to be interested are companies, organizations representing industry or civil society, individual citizens, as well as public authorities. Comments are welcomed from the EU or beyond. ECHA works with experts from the Member States to provide scientific opinions on any proposed restriction that will help the European Commission, together with the Member States, to take the final decision.
Understanding the CLP Regulation
What is the CLP Regulation?
All substances and mixtures have to be classified and any hazardous ones have to be labelled and packaged according to the Regulation on classification, labelling and packaging of substances and mixtures (CLP Regulation) before they can be placed on the market. This is important for workers and consumers who are using mixtures such as cleaning products or paints daily. Some of these contain hazardous substances that need to be handled with care and kept away from children.
The CLP Regulation entered into force on 20 January 2009. It aligns the EU legislation with the United Nations’ Globally Harmonised System (GHS). The Regulation replaced two previous pieces of legislation, the Dangerous Substances Directive and the Dangerous Preparations Directive. The internationally agreed classification criteria and labelling helps to protect human health and the environment. The standardised statements and pictograms are useful, for example, when the hazards of chemicals are communicated to workers and consumers using them.
Before placing chemicals on the market, the industry must establish the potential risks to human health and the environment of such substances and mixtures, classifying them in line with the identified hazards. The hazardous chemicals also have to be labelled according to a standardised system so that workers and consumers know about their effects before they handle them.
Thanks to this process, the hazards of chemicals are communicated through standard statements and pictograms on labels and safety data sheets. For example, when a supplier identifies a substance as “acute toxicity category 1 (oral)”, the labelling will include the hazard statement “fatal if swallowed”, the word “Danger” and a pictogram with a skull and crossbones.
When is a classification harmonised?
The most hazardous substances, in particular those that cause respiratory sensitisation, cancer, mutations, or are toxic for reproduction, have a harmonised classification. This is agreed at EU level and is legally binding for all suppliers of that substance.
EU member states, manufacturers, importers and downstream users of substances can propose that a substance has a harmonised classification if they believe it to be hazardous. EU Member States can also propose to revise an existing classification.
A public consultation is organised every time there is a proposal to harmonise the classification of a substance. The consultation lasts for 45 days and anyone, from the EU or other parts of the world, can comment. Comments are received from EU Member States, individual experts, companies and organisations representing industry or civil society.
After the consultation period, ECHA’s Committee for Risk Assessment (RAC) prepares a scientific opinion on the proposal, taking into account the received comments. The opinion has to be adopted within 18 months from the receipt of the proposal. RAC examines the evidence for all hazard classes proposed, and those that were open for commenting during the public consultation. They may also consider another category more appropriate for the classification of the substance after examining the evidence.
ECHA sends RAC’s opinions and any comments received during the public consultation to the European Commission, which decides on the proposal and updates the list of the harmonised classification in Annex VI to the CLP Regulation.
Self-classification
For most substances, the classification is not harmonised. Even when a substance has a harmonised classification, it may not be for all the hazard classes. In these cases, companies need to self-classify their substances and mixtures. It is important to collect all available information, evaluate its reliability and review it against the classification criteria before concluding on the self-classification.
Important sources of information are industry associations and the existing classifications in the C&L Inventory.
CLP – the bigger picture
CLP deals with the majority of the chemicals placed on the industrial, professional and consumer markets in the EU.
More than 20 EU laws refer to classifying and labelling chemicals, meaning that once a substance is classified, other legal requirements kick-in to control their use. These range from the requirement on employers to undertake workplace risk assessment to the possibilities to get an eco-label on a product.
When substances cannot be placed on the market for certain uses because of their classification, companies need to find safer alternatives. For example, substances which are classified as carcinogenic, mutagenic or toxic for reproduction cannot be used in mixtures supplied to consumers above certain concentration levels. A further example is that chemicals with this classification can be added to the Candidate List of substances of very high concern, which means that in the future, an authorisation may be needed for continued use of the substance. A recent example is bisphenol A, which was classified as toxic for reproduction category 1B in July 2016 and which is now proposed for the Candidate List.
Impact on companies
Chemical control legislation, like the REACH and CLP-directives, applies to almost all substances – even if not hazardous – that are placed on the European market; not only those used industrial processes but also for end-consumer use, for example in cleaning products, paints or even articles such as clothes. If you manufacture or import substances into the European Union you might have duties to observe.
Impact of REACH on companies
REACH impacts on a wide range of companies across many sectors, even those who may not think of themselves as being involved with chemicals.
To comply with the REACH regulation, companies must identify and manage the risks linked to the substances they manufacture or market in the EU. REACH places the burden of proof on companies. They have to demonstrate to the European Chemical Agency (ECHA) how the substances can be safely used, and they must communicate the risk management measures to the users.
Every manufacturer or importer of substances above 1 to/year (with a few exemptions) must submit a registration dossier to ECHA to legally place the products on the market – latest by 2018. Additionally, if a substance of very high concern (SVHC) – e.g. carcinogenic or bio-accumulative – is contained in an article above 0.1 % (w/w), a notification to ECHA is required or proof has to be shown that exposure of the substance can be excluded.
In general, under REACH you may have one of these roles:
Manufacturer: If you make chemicals, either to use yourself or to supply to other people (even if it is for export), then you will probably have some important responsibilities under REACH.
Importer: If you buy anything from outside the EU/EEA, you are likely to have some responsibilities under REACH. It may be individual chemicals, mixtures for onwards sale or finished products, like clothes, furniture or plastic goods.
Downstream users: Most companies use chemicals, sometimes even without realising it, therefore you need to check your obligations if you handle any chemicals in your industrial or professional activity. You might have some responsibilities under REACH.
Companies established outside the EU: If you are a company established outside the EU, you are not bound by the obligations of REACH, even if you export their products into the customs territory of the European Union. The responsibility for fulfilling the requirements of REACH, such as pre-registration or registration lies with the importers established in the European Union, or with the only representative of a non-EU manufacturer established in the European Union.
Impact of CLP on companies
If you are a supplier of chemicals, you must classify, label and package your substances and mixtures in accordance with the CLP Regulation. Your obligations depend upon your role in the supply chain.
Suppliers may have one or more of these roles:
- Manufacturer of substances or mixtures
- Importer of substances or mixtures
- Producer of specific articles
- Downstream user, including formulator and re-importer
- Distributor, including retailer
If you place a hazardous substance on the market, you must notify ECHA of its classification and labeling within one month of placing the substance on the market for the first time. For importers, the one month is counted from the day when a substance, on its own or contained in a mixture, is physically introduced in the customs territory of the EU.
REACH Registration Process
The Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation (EC) No. 1907/2006 demands that manufacturers or importers of most chemicals into the EU above 1 ton per year will need to register the chemicals with the European Chemical Agency (ECHA) – no matter whether the chemicals are part of a pure substance, preparation or article.
We can help you design the right registration strategy to achieve full compliance for the REACH registration process. RRC Group can assist you in assessing data requirements, evaluating data and preparing registration dossiers. For non-EU manufacturers RRC Group can also perform the role of an Only Representative; for importers or EU manufacturers, we can act as your Third-Party Representative.
Our roadmap to a successful REACH registration includes:
1. Inventory
Identification of substances, volume information, use mapping and collection of in-house data.
2. Inquiry
For phase-in substances that have not been preregistered and all non-phase-in substances an inquiry process at ECHA will be performed before placing the substances on the market for the first time.
3. Late pre-registration
If applicable a late pre-registration will be performed to benefit from an extended deadline for REACH registration in June 2018.
4. Data evaluation
Collection of existing data on the chemicals from Substance Information Exchange Fora (SIEF) and literature research.
5. Determination of data gaps
Evaluation of data gaps in the REACH registration dossier.
6. Completion of data requirements
If applicable “grouping of substances and read across” and “(quantitative) structure-activity relationship” [(Q)SAR} will be applied to fill data gaps.
7. Chemical safety assessment (CSA)/Chemical safety report (CSR)
A chemical safety assessment (CSA) needs to be completed for substances subject to REACH registration in quantities of ten tonnes or more per year. The CSA is the process that identifies the conditions under which the manufacturing and use of a substance is considered to be safe. The chemical safety report (CSR) documents the chemical safety assessment undertaken as part of the REACH registration process, and is the key source from which the registrant provides information to all users of chemicals through the exposure scenarios.
8. Preparation of a test proposal
For chemicals above 100 tonnes per year that have data gaps new tests might be necessary. Testing on vertebrate animals is the last resort for obtaining missing information on a substance and to be able to meet the information requirements of REACH. Proposals for testing need to be submitted to ECHA to check that reliable and adequate data will be produced and to prevent unnecessary animal testing.
9. Dossier preparation and submission
All data for REACH registration will be brought together in IUCLID, the registration dossier is created, and submitted to ECHA via REACH-IT.
10. Material Safety Data Sheet and CLP Labelling
A safety data sheet and CLP compliant labelling of the product containg the chemical is created.
REACH Only Representative
REACH applies only to legal entities established in the European Economic Area. Companies based outside Europe may not directly fulfill any obligations under REACH. As a consequence, customers of a non-European supplier have to fulfill all obligations under the regulations of REACH as an importer – which is a distinct disadvantage in comparison to purchasing from a European manufacturer. However, non-EU companies may appoint a European-based Only Representative (OR) to take over the tasks and responsibilities for complying with the regulations pursuant to REACH art. 8. This can simplify access to the EEA market for their products, secure the supply and reduce the responsibilities for the European customer.
Read more about the ADVANTAGES of an Only Representative.
Only representatives have to be:
- A natural person or legal entity established physically in the EEA
- Equipped with sufficient knowledge in the practical handling of the substances and information related to them
- Appointed by a mutual agreement with a manufacturer, formulator or article producer, established outside the EEA
- Responsible for complying with the legal requirements for importers under REACH
The non-Union company has to inform the importer(s) within the same supply chain of the appointment of an only representative. These importers are then regarded as downstream users for REACH.
It will be the task of the only representative to comply with all the obligations with which the importers of your products would have to comply. This includes submitting a pre-registration and a REACH registration dossier for the substance imported into the EU to the European Chemicals Agency (ECHA) before the relevant deadlines expire. It will also be the task of the only representative to keep the information available and update on i) the quantities imported and ii) the importers covered by the appointment, as well as to iii) supply the latest update of the safety data sheet. The only representative must therefore have a sufficient background in the practical handling of substances and the information related to them.
Only representatives need to be able to document for the enforcement authorities that they have been appointed by your company and for which substance and volume the appointment applies. They may also indicate in the registration dossier by whom they were appointed. They need to be able to document that the non-European company has informed the importers who are covered and who may benefit from the only representative having fulfilled the importers’ obligations.
The REACH registration by an only representative can cover volumes of a substance imported into the EU both directly from non-EU manufacturers and via non-EU distributors or formulators. Thus, as long as the non-EU manufacturer can keep track and document the channels through which the substance or mixture is imported into the EU, the volumes can be covered by the only representative. Non-EU manufacturers can change their only representative.
If you wish to make use of the only representative mechanism, please contact us.
Advantages of a REACH Only Representative
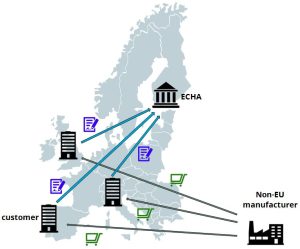
Situation without an Only RepresentativeIf a non-European manufacturer provides products to European based customers, every customer is considered to be an importer of that product. As such, the European customers of the non-EU manufacturer have to fulfill all duties of an importer pursuant to REACH art. 6 and 7.
Under REACH there are three different kind of products to be distinguished:
If the European customers buy substances on their own (above 1 to/year) every customer has to submit a registration to the European Chemical Agency to ensure legal access to the European market.
In case of supplying mixtures, the requirements apply to each individual substance (above 1 to/year) contained in the mixture. That means that every customer has to submit a registration for every single substance in the mixture.
In case of supplying articles to European customers, the customer needs information on the substances contained in the articles; in particular
In any of these cases, the customer has to submit a registration and/or notification, or has to comply with the restriction of the contained substances.
The main disadvantages of this setup:
|
 |
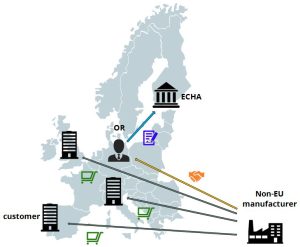
Situation after appointing an Only RepresentativeThe non-EU manufacturer may not directly fulfill any obligations under REACH as the regulation applies only to legal entities established in the European Economic Area. However, the non-EU entity – as a manufacturer, formulator of mixtures or producer of articles – may appoint a European-based Only Representative (OR) to take over the tasks and responsibilities for complying with the regulation pursuant to REACH art. 8.
The appointed Only Representative will carry out the required registration of the substance (as such, in a mixture or in an article) that is imported into the EU. This will relieve the European customers within the supply chain from their registration obligations, as they will no longer be regarded as importers but as downstream users. All information necessary to comply with REACH (for example the composition of mixtures or articles) is shared between the non-EU manufacturer and the Only Representative. Confidential data about the product does not need to be disclosed to the European customer. The main advantages of this setup:
|
 |
Who can appoint an Only Representative?
According to Article 8(1) of REACH, a natural or legal person established outside of the EU who manufactures substances (to be used on their own, in mixtures and/or to produce articles), formulates mixtures or produces articles, can nominate an only representative located within the EU to carry out the required registration of their substances that are imported (as such, in mixtures and/or in articles) into the EU. Distributors are not mentioned in Article 8(1) of REACH and thus cannot appoint an only representative.
The reference to the EU covers both the EU countries and the EFTA countries that have adhered to the EEA (European Economic Area) Agreement, that is Iceland, Liechtenstein and Norway.
What is the procedure to appoint an Only Representative?
The issue of becoming an only representative is a question of mutual agreement between the "non-EU manufacturer" and the natural or legal person established in the EU who is being appointed as an only representative.
"Non-EU manufacturers" need to send a letter confirming this appointment to their only representative who must have it available in case of inspection by the relevant Member State's enforcement authority. No such letter has to be sent to ECHA. However, when compiling the registration dossier in IUCLID 5 the only representative is advised to attach this letter of appointment to the registration dossier in the field "Official assignment from non EU manufacturer" in section 1.7.
In addition the "non-EU manufacturer" shall inform the importer(s) within the same supply chain of the appointment of the only representative according to Article 8(3) of the REACH Regulation. These importers shall be regarded as downstream users.
How can non-EU companies support their OR to prepare for registrations?
The only representative is responsible for submitting a registration dossier or a pre-registration to take advantage from the extended registration deadlines for phase-in substances. In order to assist the OR under REACH, the "non-EU manufacturer" may wish to make himself aware of the information requirements laid down in REACH and start to collect the relevant information. This may include correct identification (CAS or EINECS/ELINCS/NLP) number and naming of the substance and information on its composition.
The "non-EU manufacturer" may also assist in providing all available information regarding the intrinsic properties of the substances (see Annex VII to XI of REACH). However, these supporting measures of the "non-EU manufacturer" cannot relieve the only representative or the importer from the duty to comply with all relevant obligations of the REACH Regulation.